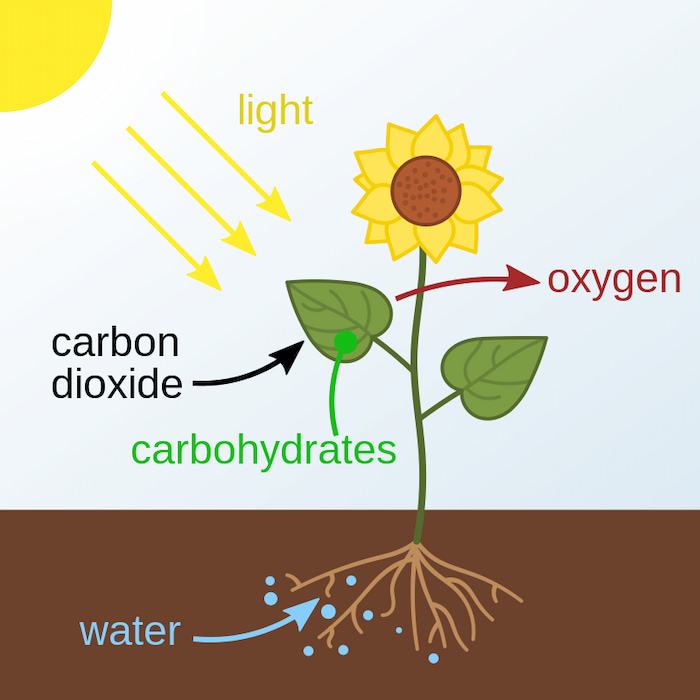
Let’s start with some basic biology. Plants use sunlight to convert carbon dioxide and water into chemical energy, producing two key products: glucose, an essential energy source for growth and reproduction, and oxygen, vital for sustaining life on Earth. This process is called photosynthesis, and its root is in Greek: phōs, "light", and synthesis, "putting together".
Since oxygen is one of the key products of photosynthesis and is vital for all respiratory processes, plants play a central role in 'fueling' aerobic life (literally meaning 'living only in the presence of oxygen'). This includes nearly all living organisms, from humans and insects to microorganisms and even plants themselves. Moreover, plants fuel the biosphere by converting sunlight into chemical energy that other organisms can access by eating them. Therefore, plants are called primary producers.
Key Learnings:
- Photosynthesis is the process by which plants convert sunlight, carbon dioxide, and water into glucose (energy) and oxygen.
- Oxygen is a crucial product of photosynthesis, vital for aerobic life on Earth.
- Glucose is stored in plants and used for growth, energy, and reproduction.
- The process occurs in two main stages: the light-dependent reactions, which produce oxygen, and the light-independent reactions (Calvin cycle), which generate glucose.
- Plants play an essential role in carbon sequestration, storing carbon, and helping mitigate climate change.
Products of Photosynthesis: A Chemical Overview
Plants and other organisms use sunlight to convert water into energy. Plants, cyanobacteria, and a variety of algae rely on photosynthesis as their primary, and often their only, source of energy, earning them the name photoautotrophs.
The Role of Chloroplasts in Photosynthesis
Plants have unique structures inside them called chloroplasts, which contain pigments sensitive to light called chromophores, such as chlorophyll. When energy is being produced, green plants, cyanobacteria or algae release oxygen during the day and take in carbon dioxide at night.
Photosynthesis Explained: What Are Its Products?
How Light-Dependent Reactions Produce Oxygen and Other Photosynthesis Products
During the daytime, plants use light energy to split water molecules apart – the light-dependent reactions. This process takes place on the thylakoid membrane, a coin-like structure inside chloroplasts. There, proteins strip electrons from water molecules, producing hydrogen ions (protons) and oxygen. This means that oxygen is a by-product of photosynthesis.
Light-Dependent Reactions: Converting Light into Energy
Oxygen is produced when light energy is absorbed by chlorophyll molecules located within the leaf cell walls. Chlorophyll molecules contain photosensitive atoms, which become excited when exposed to light. In a process called charge separation, the energy of a photon is passed onto an electron. The chlorophyll passes around the electron to a series of molecular intermediates called an electron transport chain, and eventually, after many intermediary reactions, water is oxidised, resulting in O2 and H+. This by-product of a complex reaction is the source of most oxygen in the Earth’s atmosphere.
Why Oxygen is a Crucial Product
Oxygen, one of the primary products of photosynthesis, is essential for the survival of almost all living organisms on Earth. As plants convert sunlight into energy, they release oxygen into the atmosphere, which fuels the process of respiration. For most organisms, including humans, respiration is how we extract energy from food to power our cells. Without oxygen, aerobic life would cease to exist, as it is required to produce ATP, the energy currency of cells. Beyond supporting respiration, oxygen plays a vital role in maintaining atmospheric balance and supporting diverse ecosystems. Plants, through their oxygen production, ultimately sustain life on Earth, making them central to the planet's health and survival.
The light-independent reactions
Plants use the proton generated in the light-dependent reactions to make food during the Calvin cycle. This light-independent process happens in the stroma, the fluid-filled region outside of thylakoid membranes. Since it doesn’t need light, it can theoretically take place at night and is also called the dark reactions or the light-independent reactions, but in reality, the products of the light-dependent reactions are short-lived, so the two processes are tightly coupled. The proton is then used in a different series of reactions to form two further compounds that serve as short-term stores of energy, called nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine triphosphate (ATP). Like the dollar is the currency of the US economy, ATP and NADPH are the “chemical energy currencies” of the cell’s “energy economy”.

The Calvin Cycle: Building Glucose
ATP and NADPH are used to reduce CO2, turning it into organic compounds called carbohydrates, such as glucose. These simple carbohydrates are stored as more complex starches inside the plant cells, for future use. As the plant grows, these complex carbohydrates are converted back into sugars and then used by the plant for vital functions, such as building new tissues during growth or to be used as energy during hard times, such as winter or droughts.
The oxygen is usually released shortly after being produced, during the daytime, but some plants have a delay, such as certain succulents adapted to hot deserts which only open their stomata at night to avoid damaging heat and water loss.
The Importance of Photosynthesis for the Food Chain
The glucose produced during photosynthesis forms the foundation of all food chains. Plants store this energy-rich compound as starch or convert it into other organic molecules, such as proteins and fats, which are consumed by herbivores and eventually by carnivores and omnivores. This makes plants the primary producers in nearly every ecosystem, directly or indirectly supplying the energy needed for life. Without photosynthesis, the energy flow that sustains life would come to a halt, underlining its vital role in global food production and ecological balance.
Carbon dioxide is stored by photosynthesis – but can be released
Carbon dioxide absorbed from the atmosphere is used as a building block during photosynthesis. Plants use carbon dioxide to make carbohydrates, proteins, fats, and other organic compounds. Through various processes natural or artificial processes, the resulting organic matter can be stored long-term, which is why plants and algae play a key role in carbon sequestration.
When discussing this, we must understand that ecosystems depend on complex, intertwined flows and cycles, not simple linear processes. For example, during the day plants release oxygen and consume carbon through photosynthesis, but at night their cellular respiration releases carbon back into the atmosphere. But of these two flows, the one absorbing carbon is stronger, so they are net carbon sinks and net oxygen emitters.
The Role of Photosynthesis in Carbon Sequestration
For example, we can look at wetlands: terrestrial ecosystems flooded by water for significant, recurring periods of time. They are important carbon sinks because waterlogging prevents plant material from fully decomposing. This can store the carbon for hundreds of years, and the process sequesters over 44 million tons of carbon annually. Another example is the formation of soils, which can “bury” carbon on a geological timescale. Artificial processes can also speed up carbon sequestration. By pyrolyzing biomass, humans produce biochar, which can be stored in the soil for thousands of years and could represent a significant climate change mitigation – if done sustainably.
If plants are allowed to naturally decay, however, the stored carbon is mostly released back into the atmosphere. Humans can release tremendous amounts of CO2 into the atmosphere by draining peatlands, cutting down old-growth forests and destroying grasslands, while at the same time limiting the planet’s ability to re-absorb the carbon released. Unlike natural processes, we humans have self-awareness, and the scientific understanding of the consequences of our actions.
Conclusion
As conservation enthusiasts equipped with the understanding of photosynthesis, it's our duty to ensure that the ecosystems which allow all life to eat and breathe are protected. By understanding natural cycles and how energy and matter flow between systems, we obtain the tools needed to ensure a stable climate and a world that fosters abundance for all life. Armed with this knowledge, it's our duty to act to protect the living world.
FAQ: Common Questions About Photosynthesis Products
What are the products of photosynthesis?
The main products of photosynthesis are glucose and oxygen. Glucose provides energy for plants and other organisms, while oxygen is released into the atmosphere and used by most living things for respiration.
What are two products of photosynthesis?
The two primary products of photosynthesis are glucose and oxygen. Glucose serves as an energy source for plants and other organisms, and oxygen supports aerobic life on Earth.
What is the product of photosynthesis?
Glucose is the main chemical product of photosynthesis. It is used by plants to store energy and as a building block for growth and reproduction.
What are products of photosynthesis at night?
At night, photosynthesis does not occur due to the absence of light. However, plants release carbon dioxide and consume oxygen during respiration, which is the reverse of their daytime activity.
Why is oxygen a crucial product of photosynthesis?
Oxygen, released during photosynthesis, is essential for respiration in most living organisms. It supports cellular energy production and helps maintain the atmospheric balance necessary for life on Earth.


